
Supplemental Materials for iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics - The Lancet Oncology

Cetuximab Monotherapy and Cetuximab plus Irinotecan in Irinotecan-Refractory Metastatic Colorectal Cancer | NEJM

Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry - Annals of Oncology
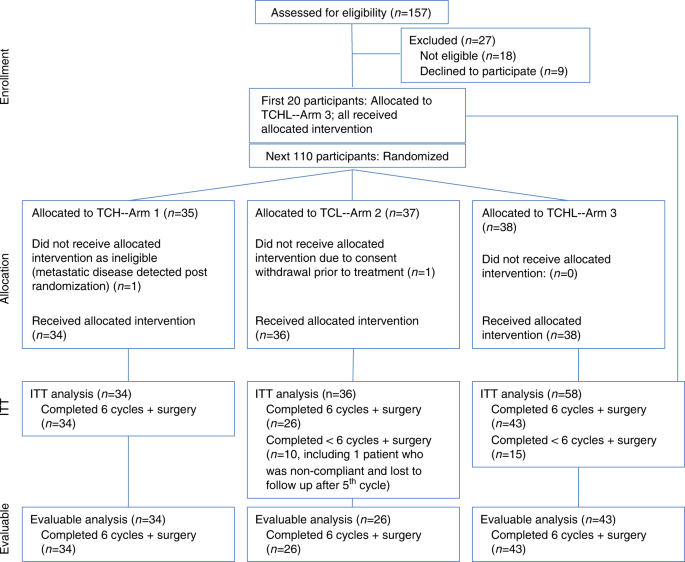
Pathologic and molecular responses to neoadjuvant trastuzumab and/or lapatinib from a phase II randomized trial in HER2-positive breast cancer (TRIO-US B07) | Nature Communications
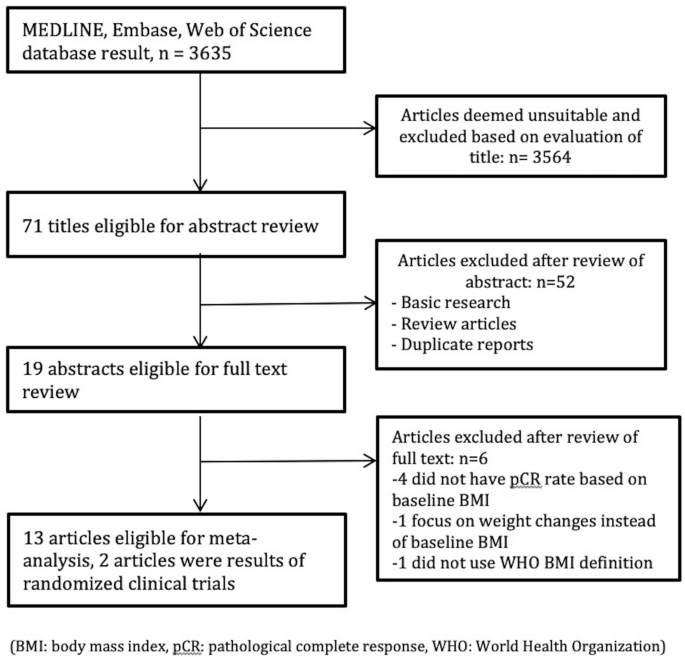
Impact of body mass index on pathological complete response following neoadjuvant chemotherapy in operable breast cancer: a meta-analysis | SpringerLink
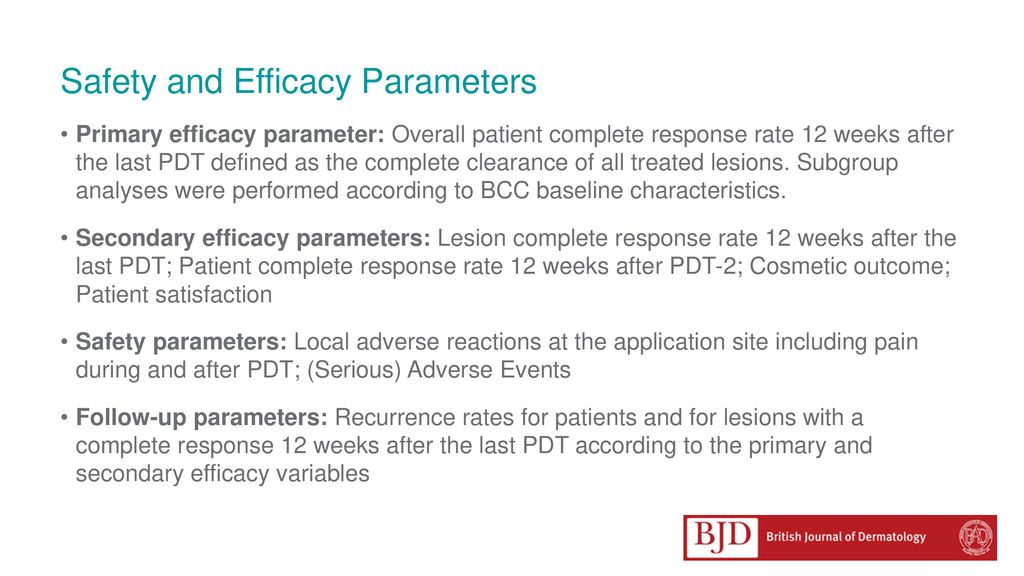
A randomized, multi-national, non-inferiority, phase III trial to evaluate the safety and efficacy of BF-200 ALA gel versus MAL cream in the treatment. - ppt download

Rituximab before splenectomy in adults with primary idiopathic thrombocytopenic purpura: a meta‐analysis - Auger - 2012 - British Journal of Haematology - Wiley Online Library
![PDF] Identification of potential surrogate end points in randomized clinical trials of aggressive and indolent non-Hodgkin's lymphoma: correlation of complete response, time-to-event and overall survival end points | Semantic Scholar PDF] Identification of potential surrogate end points in randomized clinical trials of aggressive and indolent non-Hodgkin's lymphoma: correlation of complete response, time-to-event and overall survival end points | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/1b7c7d2ac6a943299f03859ee0550a5a659900c1/4-Table2-1.png)






![PDF] Evaluation and monitoring of response to therapy in multiple myeloma. | Semantic Scholar PDF] Evaluation and monitoring of response to therapy in multiple myeloma. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/c7433976b18e415f228f8d8e6521bba0c26bbc89/2-Table1-1.png)
